
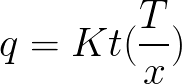
Does it feel colder? Why? If their desk is Formica laminate, then it will feel cool to their hand because the laminate is a good conductor of heat and draws heat from their hand creating a sensation of “cold” due to heat leaving the body. Once this is established, ask them to place their hand on their desk or on a metal object. Ask them if all the objects in the room are at the same temperature. Ask students what the current temperature in the classroom is.
#HEAT TRANSFER RATE SKIN#
Since the carpet and tile floor are both at the same temperature, why does one feel colder than the other? This is explained by different rates of heat transfer: The tile material removes heat from your skin at a greater rate than the carpeting, which makes it feel colder. Since the rate of heat transfer is different for different materials, we choose fabrics, such as a thick wool sweater, that slow down the transfer of heat away from our bodies in winter.Īs you walk barefoot across the living room carpet, your feet feel relatively comfortable…until you step onto the kitchen’s tile floor. Sometimes, we try to control the conduction of heat to make ourselves more comfortable. Heat transferred between the electric burner of a stove and the bottom of a pan is transferred by conduction.
#HEAT TRANSFER RATE WINDOWS#
Heat transfer by convection also occurs through cold air entering the room around windows and hot air leaving the room by rising up the chimney.Ĭonduction is heat transfer through direct physical contact.

Heat transfer also occurs through conduction into the room, but at a much slower rate. Radiation is responsible for most of the heat transferred into the room. We see from this table that the specific heat of water is five times that of glass, which means that it takes five times as much heat to raise the temperature of 1 kg of water than to raise the temperature of 1 kg of glass by the same number of degrees.įigure 11.3 In a fireplace, heat transfer occurs by all three methods: conduction, convection, and radiation. Table 11.2 gives the values of specific heat for a few substances as a handy reference. Values of specific heat must be looked up in tables, because there is no simple way to calculate them. This is because the heat capacity is a property of an object, but specific heat is a property of any object made of the same material. Consequently, two objects made up of the same material but with different masses will have different heat capacities. Note that heat capacity is the same as specific heat, but without any dependence on mass. In equation form, heat capacity C is C = m c C = m c, where m is mass and c is specific heat. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 ☌ ☌. Specific heat is closely related to the concept of heat capacity. The temperature change ( Δ T Δ T ) is the same in units of kelvins and degrees Celsius (but not degrees Fahrenheit). The specific heat c is a property of the substance its SI unit is J/(kg ⋅ ⋅ K) or J/(kg ⋅ ⋅ ☌ ☌ ). The specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ✬. The symbol c stands for specific heat, and depends on the material and phase. Where m is the mass of the substance and Δ T is the change in its temperature, in units of Celsius or Kelvin. Experiments show that the heat transferred to or from a substance depends on three factors-the change in the substance’s temperature, the mass of the substance, and certain physical properties related to the phase of the substance. One of the major effects of heat transfer is temperature change: Heating increases the temperature while cooling decreases it. There is no net heat transfer once the temperatures are equal because the amount of heat transferred from one object to the other is the same as the amount of heat returned. If two objects at different temperatures are brought in contact with each other, energy is transferred from the hotter object (that is, the object with the greater temperature) to the colder (lower temperature) object, until both objects are at the same temperature. We learned in the previous section that temperature is proportional to the average kinetic energy of atoms and molecules in a substance, and that the average internal kinetic energy of a substance is higher when the substance’s temperature is higher. Heat Transfer, Specific Heat, and Heat Capacity Check prior knowledge of conduction and convection. Review concepts of heat, temperature, and mass.


 0 kommentar(er)
0 kommentar(er)
